Abstract
Introduction: Venetoclax (Ven) is approved for relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) as monotherapy (Ven mono) or in combination (Ven paired) with rituximab based on clinical trials with selected patients (pts) and limited ibrutinib exposure. Whether Ven paired is superior to Ven mono, patterns of care, and outcomes following Ven discontinuation are unknown. Further, better delineation of adverse events (AEs) when Ven is used outside of clinical trials is needed. To address these gaps, we conducted a multicenter, international study in partnership with CLL Collaborative Study of Real World Evidence (CORE) and UK CLL Study Forum examining the clinical experience of 348 Ven treated CLL pts, representing the largest series of Ven treated pts reported to date.
Methods: We conducted a retrospective cohort analysis of CLL pts treated with Ven across 24 US and 42 UK academic and community centers. We examined demographics, baseline disease characteristics, dosing, AEs, TLS risk and outcomes, response rates, outcomes (overall survival (OS) and progression free survival (PFS)), and tx sequencing. TLS events were defined by Howard criteria. PFS and OS were estimated by the Kaplan Meier method. Comparisons of outcomes used the Log Rank test. Univariate and multivariate analyses were performed with COX regression. All other comparisons were descriptive.
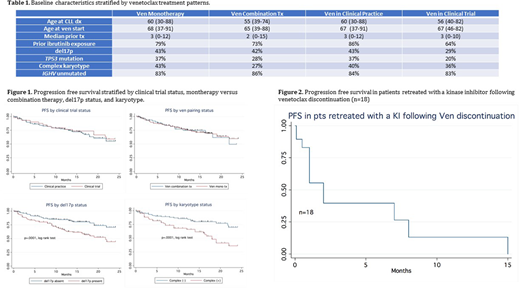
Results: Of these 348 CLL pts, 94% were R/R, median age 67 years (range:37-91), 69% male, 85% white, and 73% Rai stage ≥2. 19% received Ven on clinical trial. 79% had Ven mono; Ven was paired most commonly with anti-CD20 (n=51) and ibrutinib (n=10). Pts received a median of 3 tx (range 0-15) before Ven; 78% received ibrutinib, 29% received PI3Ki, 20% had ≥2 prior kinase inhibitors, and 68% had chemoimmunotherapy. Median time from most recent tx to Ven start was 1.1 months (range 0-62). Pre-Ven prognostic markers included 43% del17p, 34% TP53 mutated, 24% del11q, 38% complex karyotype (≥ 3 abnormalities), and 84% IGHV unmutated (Table 1). TLS risk was low in 38%, intermediate in 34% and high in 28%. During ramp up, TLS was observed in 10% (22 lab, 9 clinical TLS events, 3 missing data). Following dose escalation, 70% achieved a stable Ven dose of 400 mg, 33% required ≥ 1 dose interruption and 27% required ≥ 1 dose reduction. AEs included grade 3 neutropenia 39%, grade 3 thrombocytopenia 29%, infections 25%, grade ≥ 2 diarrhea 7.8%, and neutropenic fever 7.7%. AEs were similar whether treated on or off clinical trial. The ORR to Ven mono, Ven paired was 81% (34% CR), 86% (29% CR). With a median follow-up of 14.2 months, median PFS and OS were not reached (12 month PFS 74%, OS 82%). Figure 1 depicts PFS stratified by Ven mono vs. paired, clinical trial vs. clinical practice, del17p status, and complex karyotype. Pts who discontinued Ven due to AEs had better OS compared with those who discontinued due to progression or Richter Transformation (RT) (Median OS 47 vs. 15.1 vs. 8.6 months, respectively). In multivariate analyses, complex karyotype was the only independent predictor of PFS (HR 2.8, p <.0001) and OS (HR 3.0, p=.002). In the absence of complex karyotype, number of prior lines of tx (PFS HR 1.1, p=.03; OS HR 1.1 p=.032), presence of del17p (PFS HR 2.1 p=0.001; OS HR 1.7 p=0.03) and prior ibrutinib exposure (PFS HR 2.0, p=0.04; OS HR 1.4, p=0.3) remained independent predictors of PFS and/or OS. A total of 142 pts (41.5%) have discontinued Ven, most commonly due to CLL progression (37%), AEs (20%), and RT (10%). 67 have not had subsequent therapy. Of 75 pts treated following Ven discontinuation, most common tx was a kinase inhibitor (KI) (n= 21). Among these, 18 pts had received a KI prior to Ven and were retreated with KI (ORR 17%, median PFS 2 months, Figure 2). With limited follow up, ORR to ibrutinib post Ven in 6 KI naïve pts was 50%.
Conclusions: In this heavily pretreated, poor risk group, Ven showed favorable outcomes with comparable toxicity and efficacy on or off clinical trial. Similar outcomes were observed for Ven mono and Ven paired; longer follow up is needed from studies of Ven paired to understand depth and durability of response. Complex karyotype independently predicted inferior PFS and OS. Without complex karyotype, del(17p), multiple lines of prior tx, and prior ibrutinib tx were independent predictors of inferior outcomes. Outcomes of retreatment with KI in Ven-failure previously treated with KI were poor. Data on sequencing cellular therapies post Ven is forthcoming.
Mato:AbbVie: Consultancy, Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Research Funding; Regeneron: Research Funding; AstraZeneca: Consultancy; Medscape: Honoraria; TG Therapeutics: Consultancy, Research Funding; Acerta: Research Funding; Prime Oncology: Honoraria; Portola: Research Funding; Celgene: Consultancy; Johnson & Johnson: Consultancy. Eyre:Abbvie: Consultancy, Other: travel support; Janssen: Consultancy, Other: travel support; Roche: Consultancy; Gilead: Consultancy, Other: travel support; Celgene: Other: travel support. Nabhan:Cardinal Health: Employment, Equity Ownership. Lamanna:Acerta: Research Funding; TG Therapeutics: Research Funding; Jannsen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hill:Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Brander:Pharmacyclics, an AbbVie Company: Consultancy, Honoraria, Research Funding; Acerta: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Novartis: Consultancy, Other: DSMB; TG Therapeutics: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Teva: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Genentech: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; DTRM: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; BeiGene: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding. Barr:AbbVie, Gilead: Consultancy. Cheson:AbbVie, Roche/Genentech, Pharmacyclics, Acerta, TG Therapeutics: Consultancy. Shah:Geron: Equity Ownership; Lentigen Technology: Research Funding; Exelexis: Equity Ownership; Oncosec: Equity Ownership; Miltenyi: Other: Travel funding, Research Funding; Juno Pharmaceuticals: Honoraria. Allan:AbbVie: Membership on an entity's Board of Directors or advisory committees; Verastem: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy; Sunesis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Kennard:AbbVie, Gilead, Verastem: Consultancy. Schuster:Gilead: Membership on an entity's Board of Directors or advisory committees; Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Research Funding; Physician's Education Source, LLC: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Research Funding; Dava Oncology: Consultancy, Honoraria; OncLive: Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Skarbnik:Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Genentech: Honoraria, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau. Coombs:AROG: Other: Travel fees; Abbvie: Consultancy; Incyte: Other: Travel fees; DAVA Oncology: Honoraria; H3 Biomedicine: Honoraria. Ujjani:AbbVie: Consultancy, Speakers Bureau. Jacobs:Genentech: Honoraria. Pagel:Pharmacyclics, an AbbVie Company: Consultancy; Gilead: Consultancy. Schuh:Giles, Roche, Janssen, AbbVie: Honoraria. Shadman:Celgene: Research Funding; Gilead Sciences: Research Funding; Mustang Biopharma: Research Funding; TG Therapeutics: Research Funding; AstraZeneca: Consultancy; Pharmacyclics: Research Funding; Genentech: Research Funding; Genentech: Consultancy; Acerta Pharma: Research Funding; Beigene: Research Funding; Verastem: Consultancy; Qilu Puget Sound Biotherapeutics: Consultancy; AbbVie: Consultancy. Fox:Abbvie: Consultancy, Other: travel support, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: travel support, Speakers Bureau; Gilead: Consultancy, Other: travel support, Research Funding, Speakers Bureau; Roche: Consultancy, Other: travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Other: travel support, Speakers Bureau; Sunesis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.